- US: +1-408-610-2300
- Toll Free: +1-866-831-4085
- Become a Client
The global AI-based clinical trials solution provider market was prized by USD 1.1 billion in 2020. It is expected to observe 21.7% CAGR from 2021 to 2028.
Rising infiltration of artificial intelligence (AI), growing acceptance of AI-sourced machinery between academic world along with the pharmaceutical manufacturing companies, helpful projects via private as well as public sector, meant for R&D regarding diverse curative subjects are the factors, propelling the market for AI-based clinical trials solution provider. Rising alertness and various functions presented by artificial intelligence (AI), in the arena of clinical trials is additionally supporting the enlargement.
A move in inclination from usual procedure of medicine development to a technology sourced method, by means of main pharmaceutical companies is, furthermore, powering the market growth.
The constructive approach of these companies, in the direction of the deployment of AI sourced equipment in clinical trials plus in general medicine detection along with the improvement is expected to enhance the expansion of the AI-based clinical trials solution provider market, for the duration of the forecast.
The eruption of Covid-19 pandemic has additionally initiated an augmentation in the deployment of AI-sourced technologies. Rising acceptance of scientifically highly developed results, intended for the drug invention, improvement along with the scrutiny of enlisted information of the patients are a few factors, accountable for the rise in infiltration of AI-sourced medicine development as well as clinical trial results, throughout the Covid-19 pandemic.
|
COVID-19 Impact |
Beyond COVID-19 |
|
The AI-based clinical trial solution providers market increased by 30.7% from 2019 to 2020 |
The market is estimated to witness a y-o-y growth of approximately 20% to 25% in the next 5 years |
|
Major pharmaceutical companies are investing in artificial intelligence-based technologies through strategic initiatives that will help in boosting drug development during as well as post-pandemic. |
The COVID-19 pandemic has shifted the focus of the pharmaceutical companies, academia, CROs, and others from the traditional drug development process towards the importance of AI-based solutions for improving the clinical outcomes, curbing costs, and reducing the time of clinical trials |
|
AI-based solutions are beneficial not only in analyzing the drug development process for various therapeutic applications but also for the detection and development of a vaccine with respect to COVID-19. The pandemic has boosted AI applications for virtual screening of new chemical entities as well as repurposed drug candidates. |
Decentralized Clinical Trials (DCT) are gaining traction in this industry, mainly owing to the shifting trend toward virtual or telehealth-based clinical trials in 2020. For instance, ConcertAI, an AI-based clinical trials solution providing company, witnessed an upsurge in the use of its solution for DCT oncology trials. |
As, the pandemic caused numerous clinical trials kept on wait, key companies moved in the direction of accessible information of the patients, in that way increasing decentralized clinical trials. This has more encouraged AI-sourced solution supplier because of the efficiency of their tools in information consolidation and analysis.
Besides, AI-sourced equipment can be helpful in study the feature, concerning the Covid-19 virus for example, vaccine effectiveness and mutation. Therefore, growing use of AI-sourced result for clinical trials as well as transformed discovery throughout the pandemic is expected to additionally increase the market, throughout the forecast period.
The oncology section held the major, 24.0% revenue share and led the artificial intelligence based clinical trials solution provider market, on the basis of therapeutic applications in 2020, because of the increasing occurrences of cancer along with the rising figure of clinical trials, within the oncology subject.
Besides, the neurological diseases or conditions section is probable to observe the maximum CAGR, during the forecast period. The rising commonness of neurological complaints in addition to the increasing figure of clinical trials within the arena is the most important factors credited to the superior enlargement.
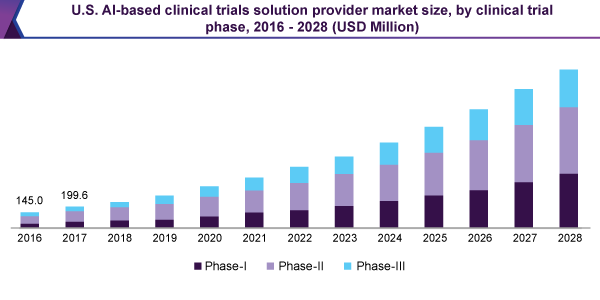
The phase-II section held the leading, 48.5% revenue share and led the AI-based clinical trials solution provider market; in 2020. This greater share is caused by the growing number of clinical trials actions, within the second phase.
Additionally, the Phase-I section is expected to develop by the highest speed, throughout the forecast period. The exercise of AI-sourced solutions as well as tools, ever since the first phase, is advantageous in retention, improved test design and patient enrollment. Therefore, its acceptance is expected to augment for the period of the forecast, by this means suggesting a sizeable speed of the enlargement.
The pharmaceutical companies section held the major, 66.9% revenue share and led the market in 2020, because of the growing acceptance of AI-sourced machinery for clinical trials and drug development by key pharmaceutical companies. Tactical programs in the structure of joint venture, mergers & acquisitions, alliances are additionally increasing the expansion of the market.
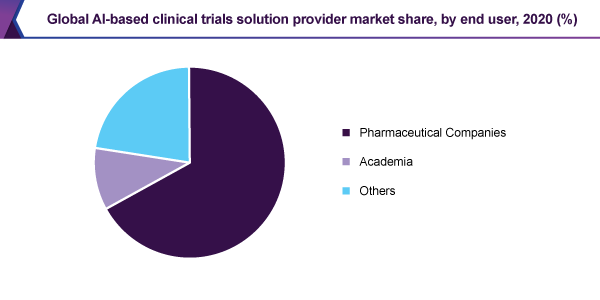
In addition, the others section is likely to view the maximum CAGR, during the forecast period. By means of the increasing utilize of artificial intelligence (AI) via pharmaceutical companies, an escalating tendency of taking on these way outs, by additional establishments like government organizations, Contract Research Organization (CROs), and the rest, pushes the expansion of the section.
In 2020, North America held the major, 43.6% revenue share and led the global AI-based clinical trials solution provider market. This enlargement of the market can be credited to diverse factors, together with rising alertness about the AI-sourced technologies and tools, a greater number of start-ups along with the artificial intelligence (AI) in drug improvement supporting companies within the region, increasing implementation of AI-sourced technologies, and rising figure of clinical trials within the region. Besides, constructive plans by the government and increasing tactical programs by major companies are pushing the demand in favor of AI-sourced clinical trials solutions, within the region.
Moreover, due to the increasing infiltration of AI-sourced tools within the region, the market in Asia Pacific is likely to display profitable enlargement, during the forecast period.
Besides, encouraging lead by the government, intended for increasing the alertness along with the acceptance of artificial intelligence (AI) in diverse healthcare areas, accompanied by drug invention plus improvement are the factors, expected to increase the market growth, during the forecast period.
The AI-based clinical trials solution provider companies working in the market have accepted the strategies such as rising R&D in the subject of artificial intelligence (AI) sourced technologies for the clinical trials, mergers & acquisitions, joint venture with new participants and alliances, with the intention of rise their market share.
• IBM Watson
• Google-Verily
• Euretos
• Trials.Ai
• Pharmaseal
• DEEP LENS AI
• AiCure, LLC
• Symphony AI
• Intelligencia
• Innoplexus
• Phesi
• Saama Technologies
• Exscientia
• GNS Healthcare
• BioSymetrics
• Koneksa Health
• Ardigen
• Halo Health Systems
• CONSILX
• BioAge Labs, Inc.
• Median Technologies
• Mendel.ai
• Deep 6 AI
• Antidote Technologies, Inc.
• Unlearn.AI, Inc.
|
Report Attribute |
Details |
|
Market size value in 2021 |
USD 1.3 billion |
|
Revenue forecast in 2028 |
USD 5.2 billion |
|
Growth Rate |
CAGR of 21.7% from 2021 to 2028 |
|
Base year for estimation |
2020 |
|
Historical data |
2016 - 2019 |
|
Forecast period |
2021 - 2028 |
|
Quantitative units |
Revenue in USD million and CAGR from 2021 to 2028 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Clinical trial phase, therapeutic application, end user, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Country scope |
U.S.; Canada; U.K.; Germany; Spain; France; Italy; Russia; China; Japan; India; South Korea; Australia; Mexico; Brazil; Argentina; South Africa; Saudi Arabia; UAE |
|
Key companies profiled |
Unlearn.AI, Inc.; Saama Technologies; Antidote Technologies, Inc.; Phesi; Deep 6 AI; Innoplexus; Mendel.ai; Intelligencia; Median Technologies; Symphony AI; BioAge Labs, Inc.; AiCure, LLC; CONSILX; DEEP LENS AI; Halo Health Systems; Pharmaseal; Ardigen; Trials.Ai; Koneksa Health; Euretos; BioSymetrics; Google- Verily; GNS Healthcare; IBM Watson; Exscientia |
|
Customization scope |
Free report customization (equivalent up to 8 analysts’ working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. |
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2016 to 2028. For the purpose of this study, Million Insights, Inc. has segmented the global AI-based clinical trials solution provider market report on the basis of the clinical trial phase, therapeutic application, end user, and region:
• Clinical Trial Phase Outlook (Revenue, USD Million, 2016 - 2028)
• Phase-I
• Phase-II
• Phase-III
• Therapeutic Application Outlook (Revenue, USD Million, 2016 - 2028)
• Oncology
• Cardiovascular Diseases
• Neurological Diseases or Conditions
• Metabolic Diseases
• Infectious Diseases
• Others
• End-user Outlook (Revenue, USD Million, 2016 - 2028)
• Pharmaceutical Companies
• Academia
• Others
• Regional Outlook (Revenue, USD Million, 2016 - 2028)
• North America
• U.S.
• Canada
• Europe
• U.K.
• Germany
• Italy
• France
• Spain
• Russia
• Asia Pacific
• Australia
• China
• Japan
• South Korea
• India
• Latin America
• Mexico
• Brazil
• Argentina
• Middle East & Africa
• South Africa
• Saudi Arabia
• UAE


Research Support Specialist, USA