- US: +1-408-610-2300
- Toll Free: +1-866-831-4085
- Become a Client
The global cell and gene therapy manufacturing market was prized by USD 13.1 billion in 2020. It is estimated to witness a 20.3% CAGR from 2021 to 2028.
The materialization of highly developed treatment has performed a key function in restructuring biopharmaceutical manufacturing, in addition, to alter the treatment pattern of several serious as well as uncommon illnesses. The substantial enlargement of the sophisticated treatment background is an input energetic factor for the escalation of the market for cell and gene therapy manufacturing.
The healthcare business is observing a better proportion of clinical accomplishment to the number of clinical trials of gene-modified treatment as well as cellular products, above the previous a hardly any years. This can be credited to the enhanced clinical plus technical understanding of security hazards, associated with the use of these products. The amount of 3rd stage clinical trials has arrived at 362 in the first part of 2020 and is anticipated to considerably boost, during the approaching years.
The current Covid-19 pandemic has completely influenced the cell and gene therapy manufacturing market. Several units extended their manufacturing capacity for the production of vectors plus made signs on the tactical accord, to create new treatments. This is to deal with the critical requirement for efficient treatments plus a vaccine, counter to the disease. For example, Telogen Bio teamed up with Bio Centriq, in March 2021, to create Tevogen’s new Covid-19 T-Cell treatment. This treatment is under evaluation, by the U.S. FDA, at present.
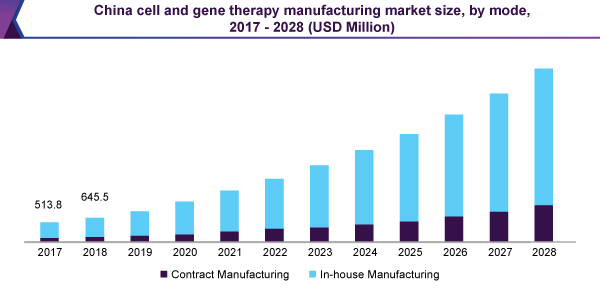
The in-house manufacturing sector held the biggest 85.0% revenue share and led the cell & gene therapy manufacturing market, in 2020. The existence of significant figures of the companies having huge funds, in addition to the educational establishments with modified plans of patient treatment, is the biggest donor to this sector.
Possession of delivery network, better alertness in troubleshooting of the procedure, and well-organized growth of a business information base, to assist upcoming domestic expansion are the input factors, increasing expansion speed of the sector.
The pre-commercial/R&D scale manufacturing sector held the biggest, 73.0% revenue share and led the market, in 2020. This can be accredited to the solid and continually increasing channel of cell and gene therapies, throughout the world.
The current eruption of SARS-CoV-2 is estimated to completely influence the enlargement of this sector. Consistent with the U.S. FDA, authorized representative organization is getting an augmented quantity of requests for the exercise of cell therapies, to deal with Covid-19.
The cell therapy manufacturing sector held the biggest, 58.0% revenue share and led the market, in 2020. The enlargement is due to the greater number of continuing clinical trials, in addition to the rising figure of the products incoming the market.
The process development sector held the biggest, 17.0% revenue share and led the cell and gene therapy manufacturing market, in 2020. Amid the rising figure of treatments progressing from clinical trials to authoritarian endorsement, the progress of perfectly distinguished as well as strong techniques for cell therapy manufacture has become ever more significant.
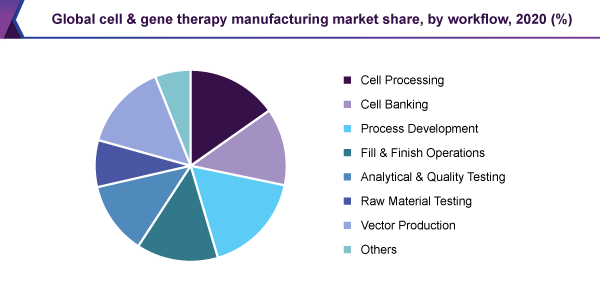
Due to the greater infiltration of manufacturing services in vector-making places, this sector is estimated to observe the highest development, during the forecast period. The viral vectors have been working for the healing of a variety of sicknesses like diverse categories of cancer as well as ophthalmologic signs, muscular, metabolic, hematologic, cardiovascular complaints, and transferable sicknesses.
In 2020, North America held the biggest, 43.0% revenue share and led the global cell & gene therapy manufacturing market. A considerable quantity of continuing clinical trials together with the increasing commitment of companies in the R&D actions of gene and cell therapy is the most important encouraging factor for the enlargement of the local market.
North America secured the foremost position for showing the maximum figure of gene therapy clinical trials by way of the existence of above 400 companies, within the region, enthusiastically busy in the expansion of cell and gene therapy products for diverse sicknesses.
Several innovative joint types of research, as well as modernization assignments, were initiated, beneath Horizon 2020 proposal, in Europe. These assignments comprise viral vector-sourced trials of gene therapy for uncommon circumstances. This is estimated to impel the enlargement of the services for cell and gene therapy manufacturing, through the nations in Europe. A well-built service set-up together with an effective labor force in European nations is expected to additionally stimulate the expansion of the market.
Conversely, the nations in the Asia Pacific are rising like solid competitors in this field. Growing needs of healthcare, the formation of stepped-up endorsement passageway, and increasing government as well as private funds, are the most important influential factors for the appearance of the market in Asia. Likewise, China is accepted as the quickly rising market for the development of cell and gene therapy and has been second positioned through the world, in the conditions of clinical trials.
The rising attraction of private as well as public financiers, in the improvement of sophisticated treatment, is expected to step up the revenue of the market for cell and gene therapy manufacturing, throughout the forecast period. Besides, the key players are taking up diverse tactical approaches like partnership, mergers & acquisitions and licensing, to increase their attendance in the market. The market has observed several prominent mergers & acquisitions, during the previous a small number of years.
• Bluebird Bio Inc.
• Cellular Therapeutics
• Novartis AG
• Samsung Biologics
• F. Hoffmann-La Roche Ltd
• Catalent Inc.
• Merck KGaA
• Miltenyi Biotec
• Hitachi Chemical Co., Ltd.
• Boehringer Ingelheim
• Wuxi Advanced Therapies
• Takara Bio Inc.
• Lonza
• Thermo Fisher Scientific
|
Report Attribute |
Details |
|
The market size value in 2021 |
USD 17.0 billion |
|
The revenue forecast in 2028 |
USD 57.4 billion |
|
Growth rate |
CAGR of 20.3% from 2021 to 2028 |
|
The base year for estimation |
2020 |
|
Historical data |
2017 - 2019 |
|
Forecast period |
2021 - 2028 |
|
Quantitative units |
Revenue in USD million and CAGR from 2021 to 2028 |
|
Report coverage |
Revenue forecast, company share, competitive landscape, growth factors, and trends |
|
Segment coverage |
Therapy type, scale, mode, workflow, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East Africa |
|
Country scope |
U.S.; Canada; Germany; U.K.; France; Italy; Spain; Japan; China; India; South Korea; Brazil; Mexico; South Africa; Saudi Arabia |
|
Companies profiled |
Thermo Fisher Scientific; Merck KGaA; Lonza; Catalent Inc.; Takara Bio Inc.; F. Hoffmann-La Roche Ltd.; Wuxi Advanced Therapies; Samsung Biologics; Boehringer Ingelheim; Novartis AG; Hitachi Chemical Co., Ltd.; Cellular Therapeutics; MiltenyiBiotec; Bluebird Bio Inc. |
|
Customization scope |
If you need specific market information, which is not currently within the scope of the report, we will provide it to you as a part of the customization |
|
Pricing and purchase options |
Avail of customized purchase options to meet your exact research needs. |
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2017 to 2028. For this study, Million Insights has segmented the global cell and gene therapy manufacturing market report based on therapy type, scale, mode, workflow, and region:
• Therapy Type Outlook (Revenue, USD Million, 2017 - 2028)
• Cell Therapy Manufacturing
• Stem Cell Therapy
• Non-Stem Cell Therapy
• Gene Therapy Manufacturing
• Scale Outlook (Revenue, USD Million, 2017 - 2028)
• Pre-commercial/ R&D Scale Manufacturing
• Commercial Scale Manufacturing
• Mode Outlook (Revenue, USD Million, 2017 - 2028)
• Contract Manufacturing
• In-house Manufacturing
• Workflow Outlook (Revenue, USD Million, 2017 - 2028)
• Cell Processing
• Cell Banking
• Process Development
• Fill & Finish Operations
• Analytical And Quality Testing
• Raw Material Testing
• Vector Production
• Others
• Regional Outlook (Revenue, USD Million, 2017 - 2028)
• North America
• U.S.
• Canada
• Europe
• Germany
• U.K.
• France
• Italy
• Spain
• The Asia Pacific
• Japan
• China
• India
• South Korea
• Latin America
• Brazil
• Mexico
• Middle East Africa (MEA)
• South Africa
• Saudi Arabia


Research Support Specialist, USA