- US: +1-408-610-2300
- Toll Free: +1-866-831-4085
- Become a Client
The global clinical trial biorepository & archiving solutions market was prized by USD 3.1 billion in 2020. It is estimated to witness 8.2% CAGR from 2021 to 2028.
Mainly, the development of the market is credited to the increasing accessibility of inexpensive computers, growth in R&D funds, increasing areas of genomic, custom-made, and accurate medicine, and a desire to make simpler information compilation along with reporting by means of the software similar to LIMS, designed for the administration of samples in laboratories.
The rising figures of companies are contracting out cold-chain and storage space procedures to ease up supply used for the pharmaceutical development. Retaining tempo with increasing transport and storage space technology, international authoritarian necessities; along with existing bio storage development can price effort and time.
Biobanking has been recognized like an important region to increase development in these businesses, because of the accessibility of translational research as well as custom-made medication. Biobanks have observed a considerable growth in the amount of samples brought together for biomarker as well as companion investigative study. The rising attraction in biomarkers to realize and recognize the organic underpinnings of illness is propelling this inclination.
On the other hand, owing to the varying authoritarian policies, lockdowns in several industrial hubs, abridged labor force, and restrictions on travel, caused by Covid-19 pandemic, disrupted the clinical trial biorepository & archiving solutions services.
The disruptions in supply chain, furthermore, caused a short of clinical trial materials and deferred deliverance of the supplies to clinical trial locations. These inconsistencies moreover produced the wastage of materials, because of the alterations in superiority of the material, which also posing like market restraints.
The phase III clinical trials sector detained the major 52.1% share of the clinical trial biorepository & archiving solutions market, in 2020. In contrast to the existing set of care, phase III clinical trials investigators evaluate the effectiveness as well as security of the latest treatment. A large amount of the patients, no less than some hundred, takes part in nearly all phase III clinical trials. Besides, owing to the huge figure of partakers in addition to the extensive period of this phase, long-standing as well as abnormal unpleasant consequences are expected more to take place.
With regards to these considerations, clinical trial biorepository & archiving solutions services are particularly significant for the phase III trials. This, necessitate an extensive variety of specimen and samples storage space for continuing research.
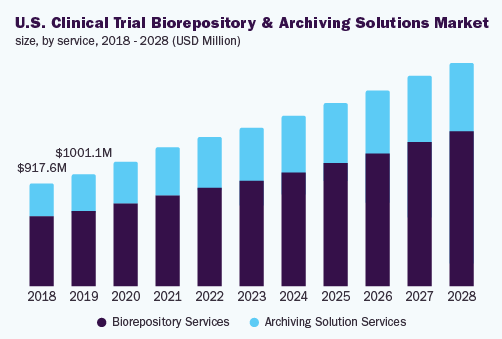
Biorepository services held 67.2% share of the market, in 2020. Strict rules like European Union General Data Protection Regulation, Right to Withdraw, Health Insurance Portability and Accountability Act (HIPAA), plus additional rules about human tissues & informed consent, access to bio specimens and sample safekeeping along with possession are expected to enhance the confidentiality of the patient plus safety of the information.
The archiving solution services section is projected to observe major development during the forecast period. The globalization of clinical trials is anticipated to activate a requirement for a number of archiving resolutions, for example multi-site archiving solutions, material indexing, tracking solutions, and real-time material management systems, by this means surely increasing the expansion of the section.
In 2020, the clinical products section held 63.1% share and dominated the market. The section is projected to record the highest expansion speed all through the forecast period. This is because of the enhancement in the amount of registrations in favor of the clinical trials, during the current years.
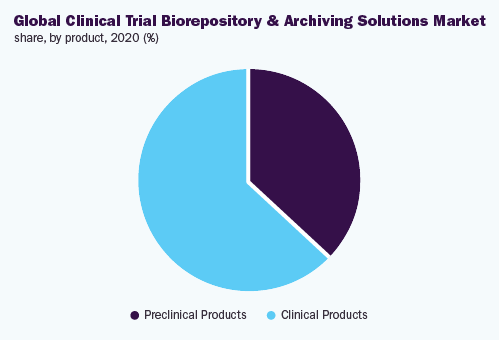
The preclinical products section is likely to observe sizeable expansion during the forecast period. The rising quantity of molecules, during the preclinical stage, is expected to generate large openings for the companies.
In 2020, North America held 49.7% share and dominated the global clinical trial biorepository & archiving solutions market. Moreover, the region is projected to develop by a major speed, throughout the forecast period.
The most important factors responsible for steering the regional market are the accessibility of contemporary technology, upsurge in the figure of clinical trials, and the existence of a huge number of companies, within the market.
Asia Pacific is expected to record the highest 15.3% CAGR, during the forecast period. The remarkable development in clinical research is likely to increase the enlargement of the local market.
In line with an assessment by Pharma IQ, 60.0% of the respondents believe India and China having the status of prospective states, in favor of clinical research, during the subsequently 5 years. The entrance of an international clinical research and manufacturing organization, Almac Group, in Asia Pacific, is an obvious sign of the expansion of the market, within this region.
The companies are taking on a variety of tactical policies, for example geographic growth, fresh business contracts, mergers & acquisitions, and partnerships, to make stronger their services along with get a viable benefit above other companies.
|
Report Attribute |
Details |
|
Market Size value in 2021 |
USD 3.4 billion |
|
Revenue forecast in 2028 |
USD 6.0 billion |
|
Growth rate |
CAGR of 8.2% from 2021 to 2028 |
|
Base year for estimation |
2020 |
|
Historical data |
2016 - 2019 |
|
Forecast period |
2021 - 2028 |
|
Quantitative units |
Revenue in USD million/billion and CAGR from 2021 to 2028 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Service, product, phase, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Japan; China; India; Australia; South Korea; Brazil; Mexico; Argentina; Colombia; South Africa; Saudi Arabia; UAE |
|
Key companies profiled |
Brooks Life Science; Patheon; Precision for Medicine, Inc.; Medpace; LabCorp Drug Development; ATCC; Q2 Solutions; Labconnect; Charles River Laboratories; Cell&Co |
|
Customization scope |
Free report customization (equivalent up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. |
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2016 to 2028. For the purpose of this study, Million Insights has segmented the global clinical trial biorepository and archiving solutions market report on the basis of service, product, phase, and region:
• Service Outlook (Revenue, USD Million, 2016 - 2028)
• Biorepository Services
• Warehousing & Storage
• Transportation
• Sample Processing
• Others
• Archiving Solution Services
• Database Indexing and Management
• Scanning & Destruction
• Product Outlook (Revenue, USD Million, 2016 - 2028)
• Preclinical Products
• Clinical Products
• Human Tissue
• Organs
• Stem Cells
• Other Biospecimens
• Phase Outlook (Revenue, USD Million, 2016 - 2028)
• Phase I
• Phase II
• Phase III
• Phase IV
• Regional Outlook (Revenue, USD Million, 2016 - 2028)
• North America
• U.S.
• Canada
• Europe
• U.K.
• Germany
• France
• Italy
• Spain
• Asia Pacific
• Japan
• China
• India
• Australia
• South Korea
• Latin America
• Brazil
• Mexico
• Argentina
• Colombia
• Middle East & Africa
• South Africa
• Saudi Arabia
• UAE


Research Support Specialist, USA