- US: +1-408-610-2300
- Toll Free: +1-866-831-4085
- Become a Client
The global clinical trial supply and logistics market was prized by USD 3.1 billion in 2020. It is anticipated to observe 7.1% CAGR from 2021 to 2028.
The enlargement of the market for clinical trial supply and logistics is credited to the increasing figure of clinical trials performed internationally, and rising R&D spending by pharmaceutical as well as biopharmaceutical companies.
Besides, generally the market is pushed by technical progressions in the supply chain. The augmented contest between companies and increasing complications in clinical analysis are the factors, accountable for the implementation of new-fangled technologies in supply chain management.
The clinical examinations have developed into progressively costly as well as composite. The medicine developers are depending on third-party service suppliers to acquire materials for clinical review and distribute them to researcher places and patients.
Additionally, dependence on contracting out for the entire clinical trial source and logistics responsibilities would propel solid development in the market. This includes transport, storage, and logistics of clinical trial supplies together with biological specimens, investigational medicines, and auxiliary materials from and to clinical locations or straight patient’s homes.
The eruption of Covid-19 pandemic presented logistical questions to the market for clinical trial, and supporters confronted several challenges in previous year. By the extensive and fast acceptance of the distant trial method, the usual supply chain has witnessed major transformations. Direct-to-patient methods were utilized to administer the variety of logistical limitations of distant trials. By way of decentralized trials, worldwide contact is extra easily attainable for the supporters. The pandemic has increased the recognition of such trials; however, demand would persist to be greater following Covid-19.
The cardiovascular diseases section detained the major, more than 25.0% revenue share of the clinical trial supply and logistics market, in 2020. This is because of the increasing and big numbers of cardiovascular investigation along with the increasing number of companies concentrated on carrying new medicines to the market.
The oncology section detained the subsequent major share, in 2020, because of the greater occurrences of cancer. Globally, cancer was the subsequent largest source of death. It was responsible for 9.6 million sufferers or one case in each six deaths. Taking in to account these factors, several pharmaceutical companies are spending on the happening of new treatments for cancer.
In 2020, phase III section detained the major, more than 40.0% revenue share of the market. Mostly, this is because of the growth in the amount of patients registered in phase III trials. In phase III analysis investigators evaluate the new treatment's effectiveness along with safety, in contrast to the existing norms of treatment. The majority phase III clinical trials include a huge figure of the patients, no less than some hundred.
Phase II clinical trials comprised the maximum amount of assignments, in 2020. This development is likely to go on because of rising funds in R&D via non-industry as well as industry supporters.
The increasing quantity of medicines in phase II is likely to amplify the difficulties in supply chain in addition to the logistics. Moreover, this is expected to augment the requirement for efficient supply chain plus logistics, in this manner encouraging the enlargement of the market.
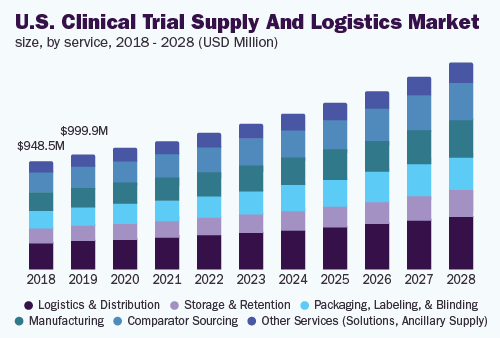
The logistics & distribution sector held more than 20.0% revenue share and dominated the market. The sector is furthermore expected to record the highest enlargement percentage, all through the forecast period.
The development in smart packaging solutions, like active along with passive temperature synchronized products guarantee that consignment stay in appropriate temperature limits whilst on the way and in storage space, shielding the filling of the consignment.
SENTRY plus additional GPS facilitated apparatuses instantaneously follow the condition and the position of the consignment. It includes disclosure with the light, motion, battery life and shock, completely whilst distributing SMS notice regarding some divergence.
The pharmaceuticals section held above 40.0% share and dominated the clinical trial supply and logistics market, in 2020, on the basis of end user. This is because of the augmented figure of clinical analysis performed by the pharmaceutical companies along with funding for R&D by these companies. The globalization of clinical trials is further sustaining the expansion of the market.
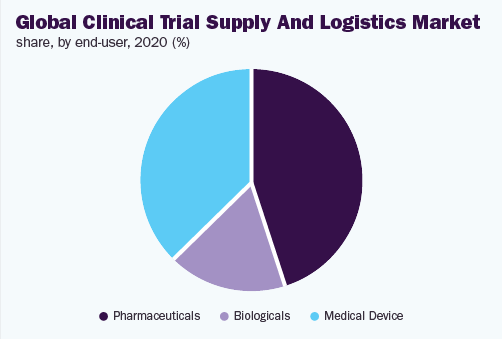
The biological section is expected to increase by the highest 7.3% CAGR during the forecast period. Mostly, this may be credited to the rising demand for biological products, like vaccines plus cell and gene treatments, along with the increasing funds for development of the product. The Covid-19 pandemic added to the expansion of the market because of the enlargement in worldwide demand for the vaccines.
In 2020, North America held more than 35.0% share of the global market. The escalation in the figure of clinical trials, carried out by pharmaceutical companies, impels the market within the region. Consistent with ClinicalTrials.gov, the U.S. makes the maximum amount of clinical trial procedures for each year. The enlargement of the local market is furthermore accredited to the existence of a large number of contestants within the market.
Asia Pacific is projected to record the highest 7.8% CAGR, during the forecast period. The region has contact to cheaper enrollment overheads, big as well as diversified patient group, plus encouraging strategies. It creates Asia Pacific attractive for the clinical reviews. Researchers are changing their concentration from urbanized nations similar to Singapore, Japan, South Korea, and Australia, to developing nations such as Vietnam, Thailand, Philippines and China.
The companies are adopting a variety of tactical approaches like geographical growth, new contracts for joint ventures and collaborations, to make stronger their services. Moreover, the companies are performing a significant function in the Covid-19 pandemic by means of giving direct-from-patient or direct-to-patient services.
Together for its conventional supply chain and direct-to-patient services, the company's supply chain makes use of packaging materials with remote temperature monitoring devices and GPS, restricted room temperature amenities, and freezers as well as refrigerators, to offer ambient temperature, cold chain, and ultra-cold chain, delivery.
|
Report Attribute |
Details |
|
Market Size value in 2021 |
USD 3.3 billion |
|
Revenue forecast in 2028 |
USD 5.4 billion |
|
Growth rate |
CAGR of 7.1% from 2021 to 2028 |
|
Base year for estimation |
2020 |
|
Historical data |
2016 - 2019 |
|
Forecast period |
2021 - 2028 |
|
Quantitative units |
Revenue in USD million/billion and CAGR from 2021 to 2028 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Service, phase, end-user, therapeutic area, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Japan; China; India; Australia; South Korea; Brazil; Mexico; Argentina; Colombia; Chile; South Africa; Saudi Arabia; UAE; Iran; Israel |
|
Key companies profiled |
Thermo Fisher Scientific (U.S.) (Patheon); Catalent, Inc. (U.S.); Parexel (U.S.); Almac Group; Marken; Piramal Pharma Solutions; UDG Healthcare; DHL; FedEx; Movianto; Packaging Coordinators Inc. |
|
Customization scope |
Free report customization (equivalent to up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail of customized purchase options to meet your exact research needs. |
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends and opportunities in each of the sub-segments from 2016 to 2028. For the purpose of this study, Million Insights has segmented the global clinical trial supply and logistics market report on the basis of service, phase, end-user, therapeutic area, and region:
• Service Outlook (Revenue, USD Million, 2016 - 2028)
• Logistics & Distribution
• Storage & Retention
• Packaging, Labeling, and Blinding
• Manufacturing
• Comparator Sourcing
• Other Services (Solutions, Ancillary Supply)
• Phase Outlook (Revenue, USD Million, 2016 - 2028)
• Phase I
• Phase II
• Phase III
• Phase IV
• End-user Outlook (Revenue, USD Million, 2016 - 2028)
• Pharmaceuticals
• Biologicals
• Medical Device
• Therapeutic Area Outlook (Revenue, USD Million, 2016 - 2028)
• Oncology
• Cardiovascular Diseases
• Respiratory Diseases
• CNS and Mental Disorders
• Others
• Regional Outlook (Revenue, USD Million, 2016 - 2028)
• North America
• U.S
• Canada
• Europe
• U.K.
• Germany
• France
• Italy
• Spain
• Asia Pacific
• Japan
• China
• India
• Australia
• South Korea
• Latin America
• Brazil
• Mexico
• Argentina
• Colombia
• Chile
• Middle East & Africa
• South Africa
• Saudi Arabia
• UAE
• Iran
• Israel


Research Support Specialist, USA