- US: +1-408-610-2300
- Toll Free: +1-866-831-4085
- Become a Client
The global COVID-19 antigen test market was prized by USD 5.3 billion in 2020. It is estimated to witness a 6.7% CAGR from 2021 to 2027.
Since it contains a smaller test-to result time span, is simple to make use of, and is patient-friendly; the COVID-19 antigen tests are increasing grip in the present situation. The scalability tests and timeline, regarding the SARS-CoV-2 PCR testing, are the most important issues, which are raising the uptake of antigen-based test approaches.
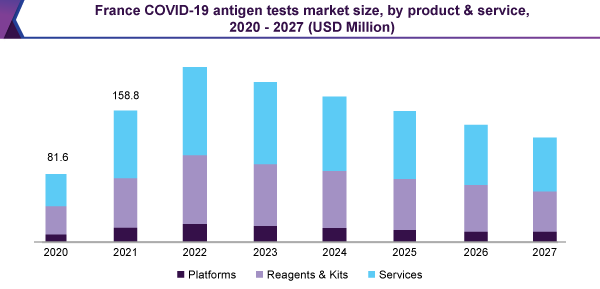
Timeline and scalability challenge, regarding SARS-CoV-2 PCR testing, are the main issues that are rising the taking up of antigen sourced test method, sequentially is encouraging the expansion of the market for COVID-19 antigen test.
The appearance of COVID-19 variants in Brazil, the U.K., and South Africa, those are discovered that these are additionally infectious than the initial strain, strengthen the want for rapid tests. Increasing apprehension above the increase and occurrence of these variants is likely to power the demand for extensive tests. As a result of this, the market is projected to observe profitable enlargement, during the close to future.
Taking into account it is like a profitable basis of revenue, during the upcoming period, a big amount of operating units transferred their focal point from usual PCR sourced testing to antigen sourced testing.
Additional factors inspiring the market for COVID-19 antigen tests comprise the scarcity of the molecular testing materials, the smaller requirement for the materials, and increasing demand for the bulk test, to restrain the increase of SARS-CoV-2, throughout the world.
The services sector held the biggest, 49.0% revenue share and led the COVID-19 antigen test market, in 2020. This can be credited to the extensive use of rapid antigen tests on diverse end-use locations like home care, clinical laboratories, and hospitals. Using the rising necessity to restrain the COVID-19 virus, a variety of service sources started presenting rapid antigen testing services.
Due to the increasing reputation of self-managed test kits, the reagents & kits sector is anticipated to develop at a considerable speed, all through the forecast period. Roche obtained a CE indication for its SARS-CoV-2 Antigen Self-Test Nasal used at-home, in June 2021. The previous type of this home test exists in the European market, from February 2021. These types of approvals are anticipated to boost the enlargement of the sector.
The clinics & hospitals division held the main, 49.3% revenue share and led the COVID-19 antigen tests market, in 2020. This end-use division observed the maximum infiltration of the rapid antigen tests, therefore, adding to the major revenue share. The U.S. FDA has accepted the antigen tests because these are appropriate for the POC analysis, intended for increasing the capability of testing, particularly in the U.S.
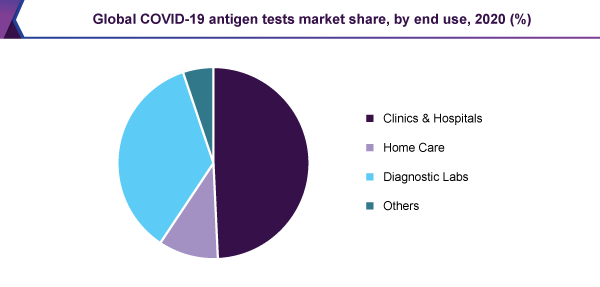
The home care sector is likely to give considerable revenue, during the approaching years. The increasing figure of over-the-counter test kits is the most important aspect for encouraging the expansion of the segment.
Pixel by LabCorp Home Collection Kit, Abbott BinaxNOW COVID-19 Antigen Self-Test, and Ellume COVID-19 Home Test Kit, are a few commercially existing Over The Counter (OTC) COVID-19 rapid antigen tests.
In 2020, Asia Pacific held the major, 37.0% revenue share and led the global COVID-19 antigen test market. The launching of numerous innovative products via rising start-ups, the existence of major regional companies, and the aggressively increasing SARS-CoV-2 antigen tests are the factors, mainly added to the supremacy of the region.
Such as, Mylab Discovery Solutions Ltd., a Pune sourced company, obtained sanction from the Indian Council of Medical Research (ICMR) for its rapid antigen test kit, in May 2021. This kit provides outcomes of the test, in 15 minutes. Moreover, the Indonesian Ministry of Health declared the exercise of rapid antigen tests, intended for the inspection of SARS-CoV-2 infectivity, in February 2021. These types of proposals are anticipated to speed up the practice of antigen tests, within the Asia Pacific.
Conversely, due to the growing figure of SARS-CoV-2 cases within the nation, the U.S. has taken on several programs, associated with antigen testing. Such as, the Centers for Disease Control and Prevention (CDC) permitted the utilization of SARS-CoV-2 antigen tests at home, used for the incoming global tourist to the U.S. These factors are anticipated to boost the exercise of antigen tests; sequentially it is increasing the revenue creation.
The market is estimated to see remarkable expansion, during the adjoining future. The rising figure of the authorizations for the usage, due to the urgent situation, plus the endorsement for the product, using authoritarian associations along with the technical partnership amid operating units are a few factors, which have strengthened the competition in the market.
• Laboratory Corporation of America
• Diasorin S.P.A
• Princeton BioMeditech Corporation
• ADS biotech Inc.
• F. Hoffmann-La Roche AG
• Mylab Discovery Solutions Pvt. Ltd
• Abbott
• Quidel Corporation
• Becton, Dickinson, and Company
• PerkinElmer, Inc.
• Access Bio. Inc.
• GenBody Inc.
• SD Biosensor Inc.
|
Report Attribute |
Details |
|
The market size value in 2021 |
USD 8.8 billion |
|
The revenue forecast in 2027 |
USD 8.3 billion |
|
Growth Rate |
CAGR of 6.7% from 2021 to 2027 |
|
The base year for estimation |
2020 |
|
Historical data |
2020 |
|
Forecast period |
2021 - 2027 |
|
Quantitative units |
Revenue in USD Million and CAGR from 2021 to 2027 |
|
Report coverage |
Revenue forecast, company share, competitive landscape, growth factors, and trends |
|
Segment coverage |
Product and service, end-use, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East Africa |
|
Country scope |
U.S.; Canada; Germany; U.K.; Italy; France; Spain; Russia; China; India; South Korea; Australia; Japan; Brazil; Mexico; South Africa; Saudi Arabia |
|
Companies profiled |
Abbott; SD Biosensor Inc.; Mylab Discovery Solutions Pvt. Ltd.; GenBody Inc.; F. Hoffmann-La Roche AG; SD Biosensor Inc.; Access Bio., Inc.; ADS biotech Inc.; PerkinElmer, Inc.; Princeton BioMeditech Corporation; Becton; Dickinson and Company; Diasorin S.P.A; Quidel Corporation; Laboratory Corporation Of America |
|
Customization scope |
If you need specific market information, which is not currently within the scope of the report, we will provide it to you as a part of the customization |
|
Pricing and purchase options |
Avail of customized purchase options to meet your exact research needs. |
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2020 to 2027. For this study, Million Insights has segmented the global COVID-19 antigen test market report based on product and service, end-use, and region:
• Product & Service Outlook (Revenue, USD Million, 2020 - 2027)
• Platforms
• Reagents & Kits
• Services
• End-use Outlook (Revenue, USD Million, 2020 - 2027)
• Clinics & Hospitals
• Home Care
• Diagnostic Labs
• Others
• Regional Outlook (Revenue, USD Million, 2020 - 2027)
• North America
• U.S.
• Canada
• Europe
• Germany
• U.K.
• Italy
• France
• Spain
• Russia
• Asia Pacific
• China
• India
• South Korea
• Australia
• Japan
• Latin America
• Brazil
• Mexico
• Middle East & Africa (MEA)
• South Africa
• Saudi Arabia


Research Support Specialist, USA