- US: +1-408-610-2300
- Toll Free: +1-866-831-4085
- Become a Client
The global immuno-oncology clinical trials market was prized by USD 5.3 billion in 2020. It is estimated to witness 13.6% CAGR from 2021 to 2028.
The growth of cancer cases, sourced by alterations in way of life due to urbanization inclined populations, rising suitability of treatment, along with the latest advancements in Immuno-oncology are the factors be credited to the enlargement of the market. Specialty pharma and small biotech are ready to perform a vital function in the expansion of the market for Immuno-oncology clinical trials.
The eruption Covid-19 pandemic had a major influence on cancer as well as clinical tests, changing treatments along with oncology patients in a number of manners. Consistent with the study by Evaluate Vantage, more than 170 surveys being stopped because of the virus. The pandemic disturbed 920 interventional oncology tests, amid February and May 2020.
Moreover, clinical trial partaking has furthermore turn into difficult because of the troubles, for example transport problem to test location or investigation sites. Moreover, a number of local establishments offered timely as well as thorough backing for virtual models and tools, demonstrating patient centric tools and consider like probable reply to this international problem.
The interventional trials held the major, 78.6% revenue share and led the immuno-oncology clinical trials market, in 2020. Approximately, there are 3042 interventional functioning clinical tests, assessing the experimental phase immune treatments, having objective of registering 5,77,076 patients.
Interventional oncology trials are intended to show that, several treatments are as effectual as the current norms of care, however by not as much of morbidity and enhanced outcomes for the cancer patients.
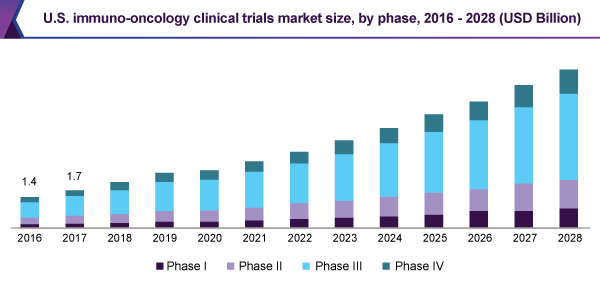
The phase III sector held 53.1% revenue share and led the immuno-oncology clinical trials market, in 2020. Mainly, this is certified that Phase III is approximately USD 59,500. Besides, oncology trials contain a lesser regular number of trials, since these are the costly ones and engage vast subject matter.
The Phase II trial section is likely to observe the highest 19.8% development percentage, during the forecast period. It is also too costliest stage, positioning Phase II following Phase III. This revision is divided into two portions. The first pace contains examination of a range of dosages with effectiveness testing, moreover the succeeding half contain choose a dosage. Phase II is significantly important, particularly in oncology tests.
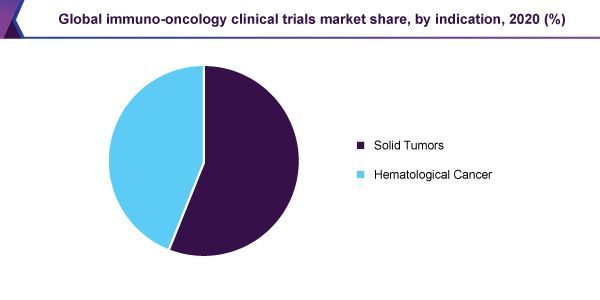
The solid tumor section held, above 56.0% revenue share and dominated the global immuno-oncology clinical trials market, on the basis of indication, in 2020. Moreover, the section is expected to observe the highest, 13.6% CAGR, for the duration of the forecast.
Progressions in cancer treatment, for example Nano medication for cancer therapy, are expected to help business participants, in offering efficient patient attention in the healing of solid tumors. On the other hand, the market for solid tumor therapy is expected to be detained via strict regulatory principles for the production of medicines.
In 2020, North America held the major, 51.0% revenue share and led the global immuno-oncology clinical trials market. This enlargement is credited to the rising demand for custom-made medication based new treatments. Besides, growing funding from the government is furthermore boosting the enlargement of the market, within the region.
Asia Pacific is projected to record the highest, 14.7% development speed, during the forecast period. The rising amount of biotechnology companies are heading for Australia as well as Asia, to carry out immune-oncology clinical studies. Esophageal cancer, bladder cancer, melanoma, stomach cancer, and lung cancer, are identified to be additional receptive to immune-oncology trials.
All over the Asia Pacific, more than 600 locations are busy in the experimental improvement of IO drugs, plus additional hundreds have added important information along with proficiency in administration of clinical trials, including immunotherapy. Frequently, these trials are carried out in the nations similar to South Korea, China, and Australia.
India is on stage, to develop into a major center for immuno-oncology clinical trials. It supposed to be regularly measured by overseas supporters, intended for utilize of Immuno-oncology (IO) medicines, for example resistant checkpoint inhibitors in medical concern along with solid tumor clinical trials.
The majority common categories of cancer documented in India comprise colorectal, lung, oral, stomach, cervical, and breast cancers. A number of private as well as government finance supported research organizations and cancer hospitals having advanced infrastructure, which is capable to deal with multi-center immune-oncology clinical trials, can be situated all over the nation.
These issues create the nation extremely lucrative. Indian authoritarian organization is accelerating the finding of new-fangled medicines, by means of accurate standards, creating Immuno-oncology clinical trials, additionally reachable.
The major companies are taking on a variety of tactical plans for example, geographic growth, making signs on the different joint venture contracts, mergers & acquisitions, to make stronger their product range, increase production capabilities, consequently presenting reasonable lead.
• Syneous Health
• Novartis
• AstraZeneca
• Exscientia
• Medpace
|
Report Attribute |
Details |
|
Market Size value in 2021 |
USD 6.2 billion |
|
Revenue forecast in 2028 |
USD 15.1 billion |
|
Growth rate |
CAGR of 13.6 % from 2021 to 2028 |
|
Base year for estimation |
2020 |
|
Historical data |
2016 - 2019 |
|
Forecast period |
2021 - 2028 |
|
Quantitative units |
Revenue in USD million and CAGR from 2021 to 2028 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Phase, design, indication, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Japan; China; India; Thailand; Singapore; Brazil; Mexico; Argentina; Colombia; South Africa; Saudi Arabia; UAE |
|
Key companies profiled |
ICON Plc; IQVIA Holdings; Covance; BioNTech; IO Biotech Medical; Medpace; Novartis; Exscientia; Syneous Health; AstraZeneca |
|
Customization scope |
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs. |
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2016 to 2028. For the purpose of this study, Million Insights has segmented the global immuno-oncology clinical trials market report on the basis of phase, design, indication, and region:
• Phase Outlook (Revenue, USD Million, 2016 - 2028)
• Phase I
• Phase II
• Phase III
• Phase IV
• Design Outlook (Revenue, USD Million, 2016 - 2028)
• Interventional trials
• Observational trials
• Expanded access trials
• Indication Outlook (Revenue, USD Million, 2016 - 2028)
• Solid tumors
• Hematological cancer
• Regional Outlook (Revenue, USD Million, 2016 - 2028)
• North America
• U.S
• Canada
• Europe
• U.K.
• Germany
• France
• Italy
• Spain
• Asia Pacific
• Japan
• China
• India
• Thailand
• Singapore
• Latin America
• Brazil
• Mexico
• Argentina
• Colombia
• Middle East & Africa
• South Africa
• Saudi Arabia
• UAE


Research Support Specialist, USA