- US: +1-408-610-2300
- Toll Free: +1-866-831-4085
- Become a Client
The global infectious disease molecular diagnostics market was prized by USD 32.4 billion in 2020. It is estimated to witness 3.0% CAGR from 2020 to 2028.
The enlargement of the market for infectious disease molecular diagnostics can be credited to the rising demand for point-of-care molecular diagnostics, the increasing utilize of molecular diagnostics in infectivity recognition, and the growing elderly populace. The eruption of Covid-19 is expected to perform like a crucial driver.
The aged populace is rising, internationally. More than 727.0 million citizens were classified in the 65 years and over age group. Due to physiological alterations linked with the age, reduced resistant performance and multi morbidity, the elderly people are extra vulnerable to Covid-19. Consistent with CDC, 80% of the Covid-19 associated deaths, in 2021, were between the citizens, having more than 65 years age. Therefore, these groups must be incessantly tested to evade acute infectivity. This generates development openings for the molecular analytic examinations, within the nations like the U.S., Russia and Japan. These nations contain the maximum aged citizens in the world.
A growth in exterior financial support to carry out clinical analysis in infectious disease molecular diagnostics is likely to steer the market.
The Human Papillomavirus (HPV) section is expected to observe rewarding 9.9% CAGR, during the forecast period. This is accredited to the augmented occurrence speed of the sickness. Escalated concentration on drug-resistant infectivity because of their increasing frequency is behind the profitable market share of the section.
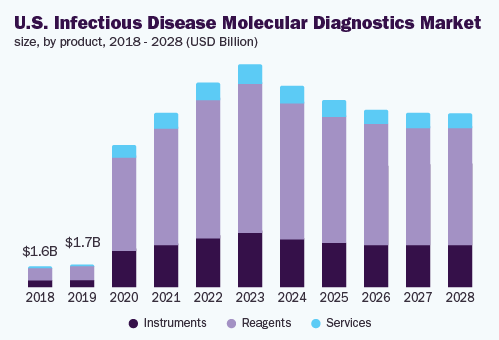
The PCR division held the biggest 90.4% revenue share and led the infectious disease molecular diagnostics market, in 2020. This enlargement can be credited to its usual application in the identification of infectious diseases plus progressions in the equipment used to distinguish new transferable sicknesses. Rising utilization of the superior throughput PCR equipment to identify corona virus, is likely to impel the expansion of the division. Besides, usual culture development processes the latest modification in DNA intensification method has initiated the induction and growth of the Transcription-mediated Amplification (TMA).
The reagents section held the major 66.7% revenue share and led the market, in 2020. It is credited to the presentation as well as commercialization of the latest reagents along with as contrast to the instruments, elevated uptake speed. Owing to escalated demand for the PCR reagents, the presentation of new reagents has substantially increased.
The instrument section is anticipated to develop by a beneficial speed, during the forecast period. In favor of increasing the range, acquirement of the companies in instrument section is growing to be a crucial policy, taken on by the key players.
As a result of greater infiltration in the market and elevated process capacity, the diagnostic laboratories division held the main 76.4% revenue share and directed the market, in 2020.
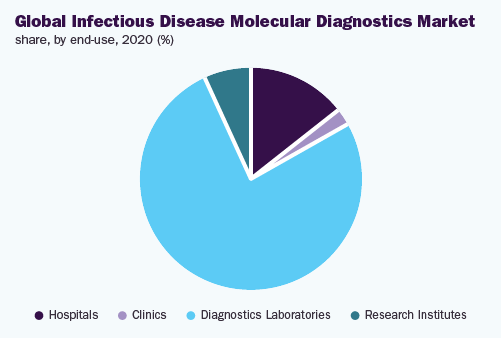
The clinics division is estimated to observe the highest development speed, during the forecast period. This is credited to first-class healthcare in addition to expediency. In addition, the patient trips to the clinics, meant for the precautionary concern, to which the transferable sickness examination is a fundamental element for the expansion of the division.
In 2020, North America held the major 34.9% revenue share and directed the global infectious disease molecular diagnostics market. This is credited to the rising authoritarian encouragement and growing finances in diagnostics, which is expected to stimulate enlargement of the market.
The rising necessity for molecular testing, to distinguish transferable sicknesses used for before time revealing of infectivity in patients, having underlying health circumstances like diabetes, plus the increasing amount of drug-resistant infectivity are the factors, expected to speed up the enlargement of the market.
As a result of increasing funds along with the progressively greater occurrences of infectious diseases, the market in Asia Pacific is projected to speedily develop during the forecast period. Constructive transformations, like the desire to get first class medicinal treatment, healthcare benefits being offered by the governments, and augmented alertness between the populations, is furthermore anticipated to impel the market, within the region.
The eruption of Covid-19 pandemic has placed huge stress on molecular diagnostics within this region, because of the thickly occupied areas and greater occurrences. Almost half of the worldwide populace lives in this area, which is headed by India and China.
The nature of this market is aggressive. Consequently, the companies are required to create novel products in terms of precision, speed, specificity, accuracy, plus additional factors, to increase a reasonable advantage.
|
Report Attribute |
Details |
|
Market size value in 2021 |
USD 40.0 billion |
|
Revenue forecast in 2028 |
USD 40.8 billion |
|
Growth Rate |
CAGR of 3.0% from 2020 to 2028 |
|
Base year for estimation |
2020 |
|
Historical data |
2017 - 2019 |
|
Forecast period |
2021 - 2028 |
|
Quantitative units |
Revenue in USD million and CAGR from 2020 to 2028 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Product, end-use, technology, application, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Country scope |
U.S.; Canada; U.K.; Germany; France; Italy; Spain; China; India; Japan; South Korea; Australia; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE |
|
Key companies profiled |
Abbott; Becton, Dickinson and Company; bioMérieux SA; Bio-Rad Laboratories, Inc.; Agilent Technologies, Inc.; Danaher Corporation; Hologic, Inc. (Gen-Probe); Illumina, Inc.; Grifols S.A.; Qiagen; F. Hoffmann-La Roche Ltd, Siemens Healthineers AG; Sysmex Corporation |
|
Customization scope |
Free report customization (equivalent to up to 8 analysts working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail of customized purchase options to meet your exact research needs. |
This report forecasts revenue growth at the global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2017 to 2028. For the purpose of this study, Million Insights has segmented the global infectious disease molecular diagnostics market report on the basis of the product, technology, end-use, application, and region:
• Product Outlook (Revenue, USD Million, 2017 - 2028)
• Instruments
• Reagents
• Services
• End-use Outlook (Revenue, USD Million, 2017 - 2028)
• Hospitals
• Clinics
• Diagnostics Laboratories
• Research Institutes
• Technology Outlook (Revenue, USD Million, 2017 - 2028)
• Polymerase Chain Reaction (PCR)
• PCR, by Type
• Multiplex PCR
• Other PCR
• PCR, by Product
• Instruments
• Reagents
• Services
• In Situ Hybridization
• Instruments
• Reagents
• Services
• Isothermal Nucleic Acid Amplification Technology (INAAT)
• Instruments
• Reagents
• Services
• Chips and Microarrays
• Instruments
• Reagents
• Services
• Mass Spectrometry
• Instruments
• Reagents
• Services
• Sequencing
• Instruments
• Reagents
• Services
• Transcription Mediated Amplification
• Instruments
• Reagents
• Services
• Others
• Instruments
• Reagents
• Services
• Application Outlook (Revenue, USD Million, 2017 - 2028)
• Respiratory Diseases
• Tuberculosis
• Meningitis
• Gastrointestinal Tract Infections
• HPV
• Sexually Transmitted Infections
• Sepsis
• Drug Resistance Diseases
• Other Infectious Diseases
• Respiratory Diseases Outlook (Revenue, USD Million, 2017 - 2028)
• Respiratory Diseases, By Product (Revenue, USD Million, 2017 - 2028)
• Instruments
• Reagents
• Services
• Respiratory Diseases, By End Use (Revenue, USD Million, 2017 - 2028)
• Hospitals
• Clinics
• Diagnostics Laboratories
• Research Institutes
• Respiratory Diseases, By Technology, (Number of Tests Performed from 2017 - 2028 in Million) (Revenue, USD Million, 2017 - 2028)
• Polymerase chain reaction (PCR)
• In Situ Hybridization
• Isothermal Nucleic Acid Amplification Technology (INAAT)
• Chips and Microarrays
• Mass Spectrometry
• Sequencing
• Transcription Mediated Amplification
• Others
• Tuberculosis Outlook (Revenue, USD Million, 2017 - 2028)
• Tuberculosis, By Product (Revenue, USD Million, 2017 - 2028)
• Instruments
• Reagents
• Services
• Tuberculosis, By End Use (Revenue, USD Million, 2017 - 2028)
• Hospitals
• Clinics
• Diagnostics Laboratories
• Research Institutes
• Tuberculosis, By Technology, (Number of Tests Performed from 2017 - 2028 in Million) (Revenue, USD Million, 2017 - 2028)
• Polymerase chain reaction (PCR)
• In Situ Hybridization
• Isothermal Nucleic Acid Amplification Technology (INAAT)
• Chips and Microarrays
• Mass Spectrometry
• Sequencing
• Transcription Mediated Amplification
• Others
• Meningitis Outlook (Revenue, USD Million, 2017 - 2028)
• Meningitis, By Product (Revenue, USD Million, 2017 - 2028)
• Instruments
• Reagents
• Services
• Meningitis, By End Use (Revenue, USD Million, 2017 - 2028)
• Hospitals
• Clinics
• Diagnostics Laboratories
• Research Institutes
• Meningitis, By Technology, (Number of Tests Performed from 2017 - 2028 in Million) (Revenue, USD Million, 2017 - 2028)
• Polymerase chain reaction (PCR)
• In Situ Hybridization
• Isothermal Nucleic Acid Amplification Technology (INAAT)
• Chips and Microarrays
• Mass Spectrometry
• Sequencing
• Transcription Mediated Amplification
• Others
• Gastrointestinal Tract Infections Outlook (Revenue, USD Million, 2017 - 2028)
• Gastrointestinal Tract Infections, By Product (Revenue, USD Million, 2017 - 2028)
• Instruments
• Reagents
• Services
• Gastrointestinal Tract Infections, By End Use (Revenue, USD Million, 2017 - 2028)
• Hospitals
• Clinics
• Diagnostics Laboratories
• Research Institutes
• Gastrointestinal Tract Infections, By Technology, (Number of Tests Performed from 2017 - 2028 in Million) (Revenue, USD Million, 2017 - 2028)
• Polymerase chain reaction (PCR)
• In Situ Hybridization
• Isothermal Nucleic Acid Amplification Technology (INAAT)
• Chips and Microarrays
• Mass Spectrometry
• Sequencing
• Transcription Mediated Amplification
• Others
• HPV Outlook (Revenue, USD Million, 2017 - 2028)
• HPV, By Product (Revenue, USD Million, 2017 - 2028)
• Instruments
• Reagents
• Services
• HPV, By End Use (Revenue, USD Million, 2017 - 2028)
• Hospitals
• Clinics
• Diagnostics Laboratories
• Research Institutes
• HPV, By Technology, (Number of Tests Performed from 2017 - 2028 in Million) (Revenue, USD Million, 2017 - 2028)
• Polymerase chain reaction (PCR)
• In Situ Hybridization
• Isothermal Nucleic Acid Amplification Technology (INAAT)
• Chips and Microarrays
• Mass Spectrometry
• Sequencing
• Transcription Mediated Amplification
• Others
• Sexually Transmitted Infections Outlook (Revenue, USD Million, 2017 - 2028)
• Sexually Transmitted Infections, By Product (Revenue, USD Million, 2017 - 2028)
• Instruments
• Reagents
• Services
• Sexually Transmitted Infections, By End Use (Revenue, USD Million, 2017 - 2028)
• Hospitals
• Clinics
• Diagnostics Laboratories
• Research Institutes
• Sexually Transmitted Infections, By Technology, (Number of Tests Performed from 2017 - 2028 in Million) (Revenue, USD Million, 2017 - 2028)
• Polymerase chain reaction (PCR)
• In Situ Hybridization
• Isothermal Nucleic Acid Amplification Technology (INAAT)
• Chips and Microarrays
• Mass Spectrometry
• Sequencing
• Transcription Mediated Amplification
• Others
• Sepsis Outlook (Revenue, USD Million, 2017 - 2028)
• Sepsis, By Product (Revenue, USD Million, 2017 - 2028)
• Instruments
• Reagents
• Services
• Sepsis, By End Use (Revenue, USD Million, 2017 - 2028)
• Hospitals
• Clinics
• Diagnostics Laboratories
• Research Institutes
• Sepsis, By Technology, (Number of Tests Performed from 2017 - 2028 in Million) (Revenue, USD Million, 2017 - 2028)
• Polymerase chain reaction (PCR)
• In Situ Hybridization
• Isothermal Nucleic Acid Amplification Technology (INAAT)
• Chips and Microarrays
• Mass Spectrometry
• Sequencing
• Transcription Mediated Amplification
• Others
• Drug Resistance Disease Outlook (Revenue, USD Million, 2017 - 2028)
• Drug Resistance Disease, By Product (Revenue, USD Million, 2017 - 2028)
• Instruments
• Reagents
• Services
• Drug Resistance Disease, By End Use (Revenue, USD Million, 2017 - 2028)
• Hospitals
• Clinics
• Diagnostics Laboratories
• Research Institutes
• Drug Resistance Disease, By Technology, (Number of Tests Performed from 2017 - 2028 in Million) (Revenue, USD Million, 2017 - 2028)
• Polymerase chain reaction (PCR)
• In Situ Hybridization
• Isothermal Nucleic Acid Amplification Technology (INAAT)
• Chips and Microarrays
• Mass Spectrometry
• Sequencing
• Transcription Mediated Amplification
• Others
• Infectious Disease Molecular Diagnostics Regional Outlook (Revenue, USD Million, 2017 - 2028)
• North America
• U.S.
• Canada
• Europe
• U.K.
• Germany
• France
• Italy
• Spain
• Asia Pacific
• Japan
• China
• India
• South Korea
• Australia
• Latin America
• Brazil
• Mexico
• Argentina
• Middle East & Africa
• South Africa
• Saudi Arabia
• UAE


Research Support Specialist, USA