- US: +1-408-610-2300
- Toll Free: +1-866-831-4085
- Become a Client
The global medical device testing services market was prized by USD 7.8 billion in 2020. It is estimated to witness an 11.6% CAGR from 2021 to 2028.
The rising pressure of the stringent rules, imposed by the government has initiated the rising demand for confirmation & corroboration for medical devices, mainly these are the factors, driving the market for medical device testing services.
Moreover, the growth in the figure of minor medical device manufacturing companies, which needs internal proficiency, is additionally increasing the enlargement of the market. The current technical improvement in medicinal manufacturing is, moreover, expected to contain an encouraging influence on the requirement for the testing services, throughout the forecast period.
Progressions in the enhancement as well as the standardization of the new in-vitro investigation technique, mainly for irritation, sensitization, and cytotoxicity, are furthermore expected to impel the expansion of the medical device testing services market. These are a few, mainly fundamental tests, necessary for every medical device.
The nation-wise guiding principles for medical devices change, and each manufacturing company must track these procedures, whilst promoting their products in a specific nation-state. For example, Europe thinks about CE endorsement, the U.S., go after FDA guidelines, India has to need CDSCO authorization, and Canada necessitates Health Canada Registration. Therefore, these dissimilar sets of laws, in diverse nation-states, make it difficult to instigate a product on an international level.
The outbreak of the Covid-19 pandemic has triggered a gush in demand for medical device testing. However, as the supply of medical devices is in short, the majority of medical equipment that needs to be tested is Personal Protective Equipment (PPE). PPE testing includes authorizing their use again, together with single-use masks. In anticipation of the inception of the pandemic, the majority of facemasks were proposed like single-use products.
Yet, due to their scarcity, all over the world, many manufacturing companies are functioning on alternatives to check these masks for multiple uses. The assessment comprises sterilization tests, fitment tests, and obedience to initial filtration terms.
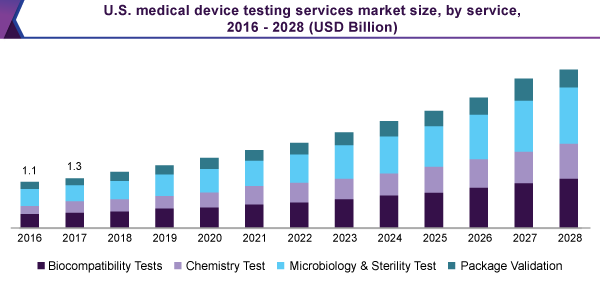
The microbiology and sterility testing sector held the major 34.1% revenue share, in 2020, and led the medical device testing services market. The section is additionally subdivided into anti-microbial activity testing, Pyrogen, & Endotoxin testing, bioburden determination, and sterility test & validation.
Microbiology and sterility testing is carried out to eradicate or decrease the threat of contagion in the manufacturing procedure. This can source, infection in the patients otherwise the users of products. Falling short in completing such assessment may holdup the authoritarian procedure for the devices.
The package validation sector guarantees the reliability of the product and defends counter to the possible damages, all through the supply chain. Even though the market is not as much monopolized, and held 14.0% of the revenue shares in 2020, the manufacturing companies are, at present, taking into account the financial as well as ecological aspects, to revalidate their wrapping.
Besides, authoritarian establishments like FDA have augmented their concentration on the packaging of medical devices. It is performing like a pushing power at the back of the market expansion.
In 2020, the preclinical phase held the principal, above 60.0% revenue share, and dominated the medical device testing services market. Since the main part of chemistry, biocompatibility, and microbiology check are carrying out within this phase the sector is, moreover, expected to lead the market, during the forecast period.
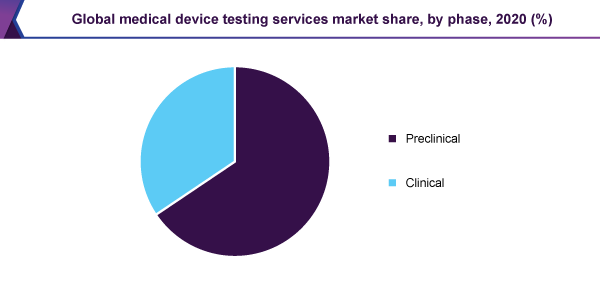
Since the medical devices are essential and they contain a straight influence on human life, medical device testing services, for the duration of the clinical trial phase, are anticipated to considerably increase, during the near future. Therefore, the manufacturing companies of the medical devices are necessary to stick to the testing, together with authentication & corroboration practices, to make sure the excellence & dependability of the medical devices. This is expected to add to the expansion of the market for medical device testing services.
In 2020, Asia Pacific held the major 41.8% revenue share and dominated the medical device testing services market. Owing to the rising worldwide attraction for India and China markets, strict rules for the product sanction in the nations like China, and the progression in healthcare infrastructure, the region is, furthermore, anticipated to develop at a momentous speed, in the forecast period. China is the most important exporter for numerous companies, all over the world. This has given rise to a boost in medical device testing services, within the nation, to retain fulfillment with global standards.
The market in North America, held the subsequent largest revenue share, in 2020. It is estimated to speedily develop, throughout the forecast period. Increasing efforts regarding cutting the costs and growing complications in designing the product are the most important issues, pushing this local market. In addition, the existence of strict authoritarian organizations like the FDA is powering the enlargement of the market.
The companies are taking on a variety of tactical programs like mergers & acquisitions, joint ventures, geographical growth, and the starting of innovative services, intended to make stronger their range of the product and give a reasonable benefit.
• Medical Device Testing Services
• Sterigenics International LLC
• TÜV SÜD AG
• Wuxi AppTec
• Pace Analytical Services LLC
• Toxikon, Inc.
• Charles River Laboratories International, Inc.
• American Preclinical Services
• North American Science Associates, Inc.
• Intertek Group Plc
• Euro fins Scientific
• SGS SA
|
Report Attribute |
Details |
|
The market Size value in 2021 |
USD 8.7 billion |
|
The revenue forecast in 2028 |
USD 18.6 billion |
|
Growth Rate |
CAGR of 11.6% from 2021 to 2028 |
|
The base year for estimation |
2020 |
|
Historical data |
2016 - 2019 |
|
Forecast period |
2021 - 2028 |
|
Quantitative units |
Revenue in USD Million and CAGR from 2021 to 2028 |
|
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
|
Segments covered |
Service, phase, region |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
|
Country scope |
U.S.; Canada; U.K.; Germany; France; Italy; Spain; Netherlands; Belgium; Switzerland; Russia; Sweden; Japan; China; India; Australia; South Korea; Malaysia; Indonesia; Singapore; Philippines; Thailand; Brazil; Mexico; Argentina; Colombia; Chile; South Africa; Saudi Arabia; UAE; Israel; Egypt |
|
Key companies profiled |
SGS SA; Toxikon, Inc.; Eurofins Scientific; Pace Analytical Services LLC; Intertek Group Plc; Wuxi AppTec; North American Science Associates, Inc.; TÜV SÜD AG; American Preclinical Services; Sterigenics International LLC; Charles River Laboratories International, Inc.; Medical Device Testing Services |
|
Customization scope |
Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope. |
|
Pricing and purchase options |
Avail of customized purchase options to meet your exact research needs. |
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2016 to 2028. For this study, Million Insights has segmented the medical device testing services market report based on service, phase, and region:
• Service Outlook (Revenue, USD Million, 2016 - 2028)
• Biocompatibility Tests
• Chemistry Test
• Microbiology & Sterility Test
• Bioburden Determination
• Pyrogen & Endotoxin Testing
• Sterility Test & Validation
• Antimicrobial Activity Testing
• Others
• Package Validation
• Phase Outlook (Revenue, USD Million, 2016 - 2028)
• Preclinical
• Clinical
• Regional Outlook (Revenue, USD Million, 2016 - 2028)
• North America
• U.S.
• Canada
• Europe
• U.K.
• Germany
• France
• Italy
• Spain
• Netherlands
• Belgium
• Switzerland
• Russia
• Sweden
• The Asia Pacific
• Japan
• China
• India
• Australia
• South Korea
• Malaysia
• Indonesia
• Singapore
• Philippines
• Thailand
• Latin America
• Brazil
• Mexico
• Argentina
• Colombia
• Chile
• Middle East & Africa
• South Africa
• Saudi Arabia
• UAE
• Israel
• Egypt


Research Support Specialist, USA